Featured
A Compound That Produces Hydrogen Ions In Solution Is A
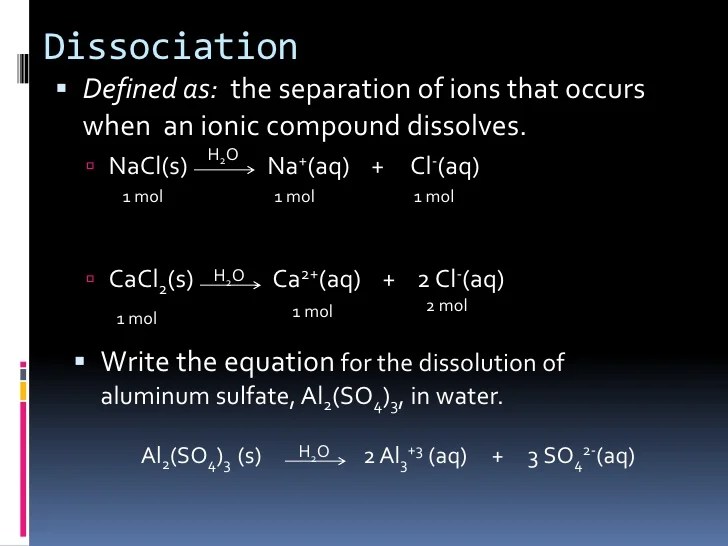
A Compound That Produces Hydrogen Ions In Solution Is A. A compound which ionizes to yield hydrogen ions (h+) in aqueous solution. Phosphoric acid is the most common substance that produces three hydrogen ions in solution.
The particle formed when a base gains a hydrogen ion; The ph scale ranges from 0 to 14, with a ph of 7 being neutral. Ka the ratio of the concentration of the dissociated form of an acid to the undissociated form;
This Is A Reversible Reaction.
A base is an ionic compound that produces negative hydroxide ions when dissolved in water.bases taste bitter and turn red litmus paper blue. Acid “an acid is a substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous solutions. What compound produces three hydrogen ions in a solution?
According To Arrhenius, An Acid Is A Compound That.
An acid is an ionic compound that produces positive hydrogen ions when dissolved in water. Phosphoric acid is the most common substance that produces three hydrogen ions in solution. A compound which ionizes to yield hydrogen ions (h+) in aqueous solution.
Ions Are Produced In Any Solution Only, When The Added Electrolyte Is Of The Same Type As That Of The Solution, Because Like Dissolves Like.
What compound produces h+ ions in water? An acid produces h+ ions in solution. Ka the ratio of the concentration of the dissociated form of an acid to the undissociated form;
The Higher The Hydrogen Ion Concentration, The More Acidic The Solution And The Lower The Ph.
What produces h+ ions in water? A (n) base is a compound that produces hydroxide ions when dissolved in water. What is the name of the compound that form hydrogen ions in aqueous solution?
The Dissociation Of Any Compound Produces Various Ions In Solutions.
A solution with a high number of hydroxide ions is basic and has a high ph value. A (n) _____ is a compound that produces hydrogen ions when dissolved in water. Some of these hydrogen and hydroxide ions then react together again to form water molecules.
Comments
Post a Comment