Featured
Acidity Of Aromatic Compounds
Acidity Of Aromatic Compounds. 5 key factors that affect acidity in organic chemistry. Aromatic carboxylic acids show not only the acidity and other reactions expected of carboxylic acids (as an acid, benzoic acid is slightly stronger than acetic acid) but, similar to other aromatic compounds, also undergo electrophilic substitution.
The pka of benzoic acid, c6h 5co2h, is known and widely reported, pka = 4.202. Aromatic compounds can also be degraded anaerobically, via reductive pathways, which have been reviewed. Learn aromatic compounds topic of chemistry in details explained by subject experts on vedantu.com.
The Simplest Aromatic Acid Is Benzoic Acid.
They are known as aromatic due to their pleasant smell. They react with metals and alkalis to generate carboxylate ions. Thus, if you didn’t pay attention to.
Aromatic Acids Include Compounds That Contain A Cooh Group Bonded To An Aromatic Ring.
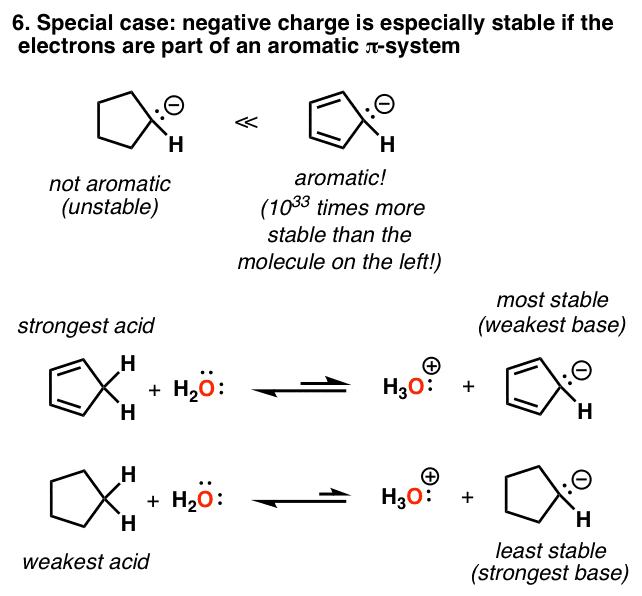
• its acidity can be traced to the strong stabilization of the resulting negative charge because the resulting pi system has an. Acidity से कैसे पाएं छुटकारा ? Carboxylic acid as an acid:
The Acidity Of Carboxylic Acids Is Higher In Comparison To Simple Phenols As.
Another interesting point about this reaction is. To make tosylic acid you just sulfonate the toluene and you got it. Asked aug 26, 2017 at 21:11.
Consider The Lone Pairs Of Electrons.
There are several categories of aromatic acids including: Register free for online tutoring session to clear your doubts. As i said, aromatic compounds are not naturally acidic.
Reactions Of Aromatic Compounds 4 Topics.
Aromatic carboxylic acids show not only the acidity and other reactions expected of carboxylic acids (as an acid, benzoic acid is slightly stronger than acetic acid) but, similar to other aromatic compounds, also undergo electrophilic substitution. Out of formic acid, acetic acid and benzoic acid, which carboxylic acid is most acidic? Sulphamethoxazole, are used as antimicrobial agents.
Comments
Post a Comment